SCADA stands for Supervisory Control and Data Acquisition. It is a system used in manufacturing and various industrial operations for monitoring and controlling processes. Here’s a breakdown of what SCADA entails:
Read MoreCategory: LEARNING
Selecting the best MES Solution for your Life Sciences Business. What questions would you ask?
Scenario: You work in the life sciences industry and get an opportunity to help your employer choose the best MES Solution for your business. If you could ask vendors within the industry a select set of questions about their products for a side-by-side comparison… What questions would you like answered?
Read MoreWhy Chinese Hamster Ovaries are used in the pharmaceutical industry?
Chinese hamster ovary (CHO) cells are commonly used in the pharmaceutical industry for the production of therapeutic proteins. There are several reasons for this: It is important to note that while CHO cells are widely used, other cell lines such as human embryonic kidney (HEK) cells and insect cells (baculovirus expression system) are also utilized in the pharmaceutical industry for protein production. The choice of cell line depends on factors such as the nature of the protein being produced, production requirements, and regulatory considerations.
Read MoreUpstream vs Downstream – Pharmaceutical Manufacturing Explained
Upstream and downstream are terms used in pharmaceutical manufacturing to describe different stages of the production process. Upstream refers to the initial stages of manufacturing, which involve the cultivation and growth of cells or organisms that produce the desired drug or therapeutic substance. This can include activities such as cell line development, fermentation, and primary purification. Downstream refers to the subsequent stages of manufacturing, which involve the purification, separation, and processing of the drug substance obtained from upstream processes. Downstream activities typically include filtration, chromatography, crystallization, and formulation. In summary,…
Read MoreUsing IIoT and AI in Manufacturing
IIoT (Industrial Internet of Things) refers to the network of interconnected devices, sensors, and machines in industrial settings. It enables the collection, analysis, and sharing of data to improve efficiency, productivity, and decision-making. AI (Artificial Intelligence) can be integrated with IIoT to enhance manufacturing processes in several ways: In summary, the combination of IIoT and AI in manufacturing allows for improved predictive maintenance, enhanced quality control, optimized production, intelligent automation, and product innovation. This integration can drive operational excellence, cost savings, and competitive advantages in the manufacturing industry. OPEN Source…
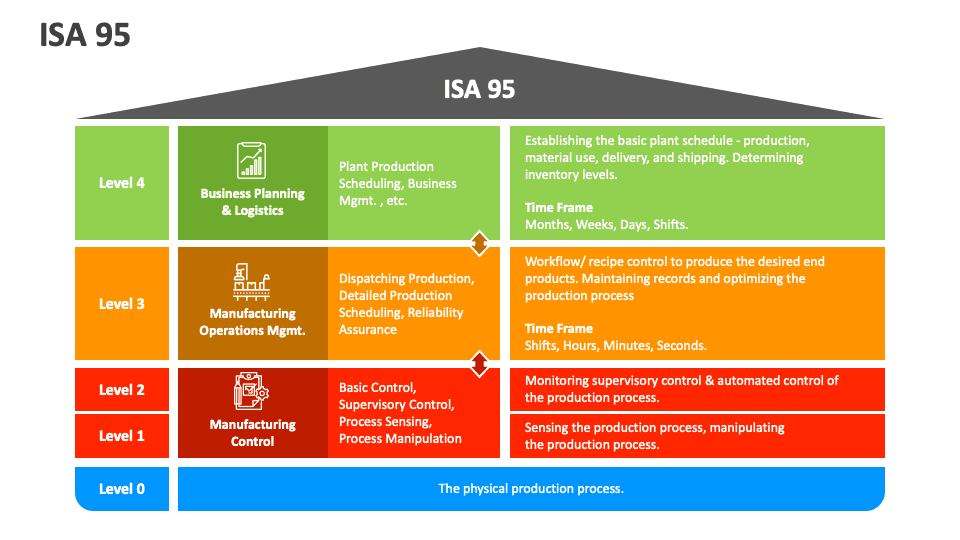
Read MoreISA-95 Explained
ISA-95, also known as the International Society of Automation standard, is a comprehensive framework that provides guidelines for integrating different systems and processes within the manufacturing industry. The standard focuses on improving communication, data exchange, and interoperability between various levels of an organization, such as the control level, operations level, and business level. The ISA-95 standard is divided into a hierarchical model that consists of four levels: ISA-95 provides a common language and set of guidelines for data exchange and integration between these levels. It enables manufacturers to integrate different…
Read MoreBasics of setting up a F5 Load Balancer
To set up an F5 load balancer with health monitoring, port-based load balancing, and session persistence, you can follow these steps: Please make sure to consult the official F5 documentation for detailed instructions specific to your F5 load balancer model and firmware version.
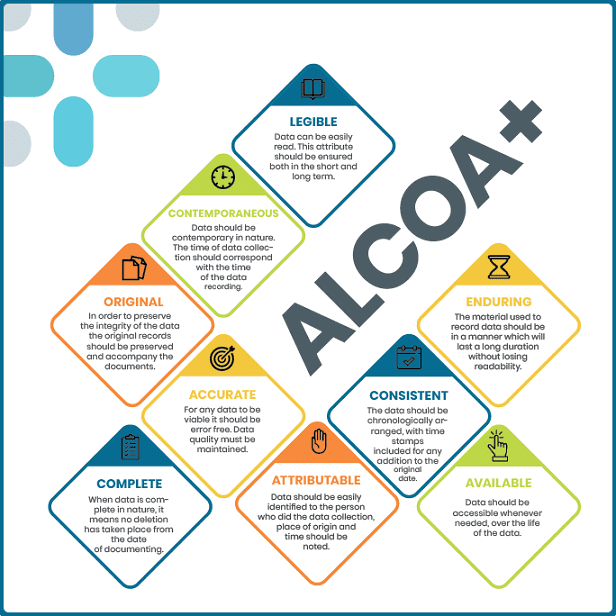
Read MoreALCOA+
ALCOA+ is a quality management framework used in various industries, particularly in healthcare and clinical research. It is an extension of the original ALCOA principles, which are a set of guidelines to ensure data integrity in regulated environments. ALCOA stands for: The “+” in ALCOA+ represents additional principles that enhance data integrity. These principles include: Adhering to the ALCOA+ framework helps organizations ensure the reliability and trustworthiness of their data by maintaining accurate, complete, and unalterable records. By following these principles, organizations can improve data quality, transparency, and compliance with…
Read MoreCommon Biotech Buzzwords and Descriptions
Buzzword Description Gene Editing The process of modifying an organism’s genetic material to achieve desired traits or characteristics. It involves targeted changes to the DNA sequence, such as adding, deleting, or modifying specific genes. CRISPR A revolutionary gene-editing technology that allows scientists to precisely modify an organism’s DNA. CRISPR-Cas9, the most widely used CRISPR system, uses a molecular tool to guide the editing process by directing the Cas9 enzyme to cut the DNA at specific locations, enabling the addition, removal, or modification of genetic material. Biopharmaceuticals Medicinal products derived from…
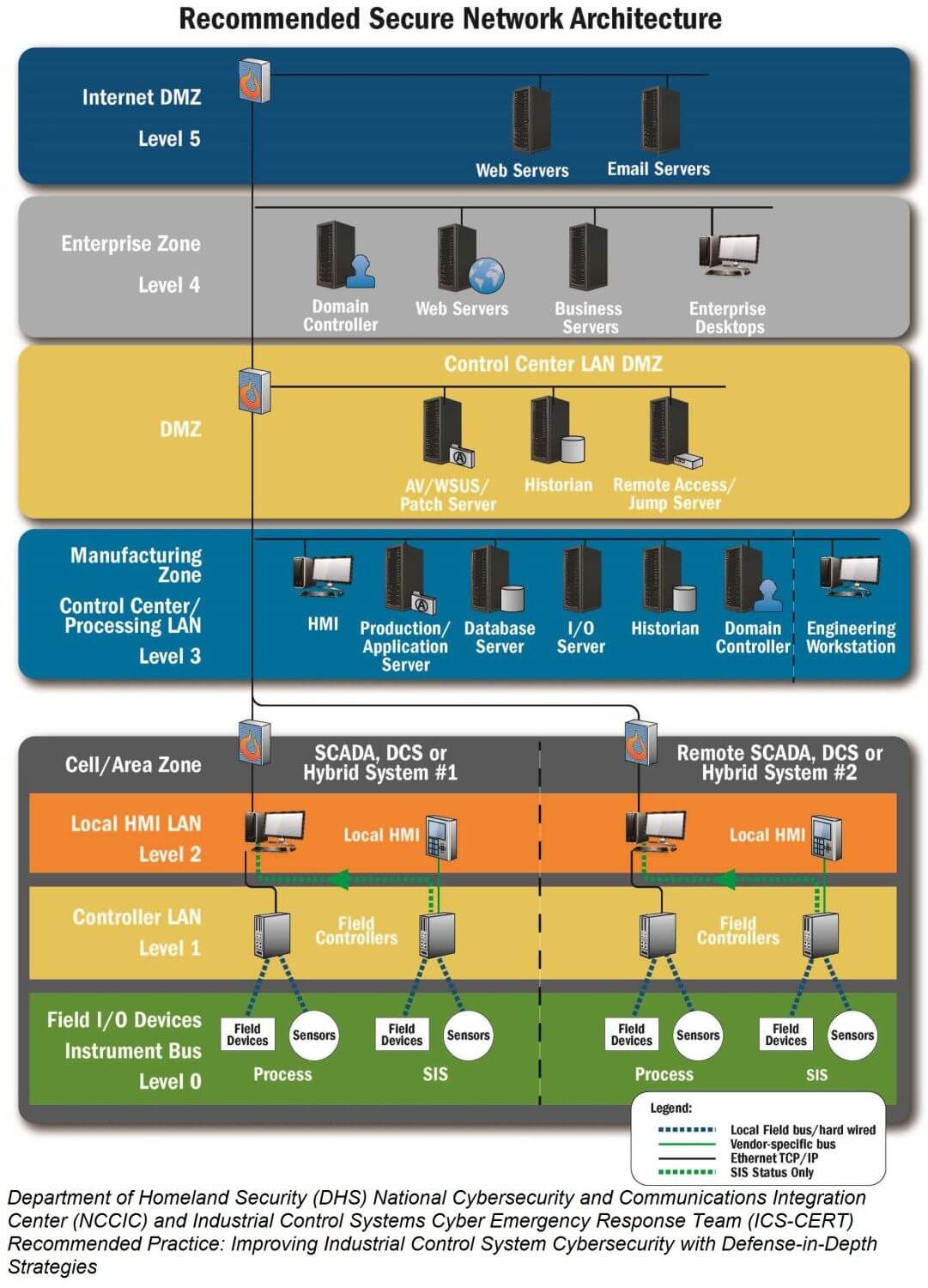
Read MoreThe Purdue Model of Security for Manufacturing: Safeguarding Industrial Control Systems
In an increasingly interconnected world, securing industrial control systems (ICS) has become a critical concern for manufacturing companies. One widely recognized approach to ICS security is the Purdue Model, also known as the Purdue Enterprise Reference Architecture. Developed by the Purdue University’s Center for Education and Research in Information Assurance and Security (CERIAS), this model provides a framework for organizing and securing control systems in manufacturing environments. Understanding the Purdue Model The Purdue Model is based on the concept of hierarchical levels, each responsible for specific functions and security controls…
Read More